Projects
LRX1 as a model to investigate LRX function
(Baumberger et al., Plant Journal, 2003)
LRXs consist of an LRR domain and an extensin domain. The LRR domain has been shown to interact with RALFs (Rapid Alkalinization Factor), peptide hormones that influence cell growth processes. Recent work has demonstrated a pectin-compacting function of LRX-RALF complexes, suggesting a direct effect of LRXs on cell wall structures. In the absence of LRXs, plant cells are less responsive to the growth-inhibiting effect of RALFs and show aberrant cell wall formation. LRX proteins function in conjunction with FERONIA, a plasma membrane-localized receptor kinase. In the absence of LRXs or FER, cells are impaired in sensing alterations in cell wall composition or detecting salt stress, implying that LRX and FER are important for the exchange of information during cell wall integrity sensing.
We believe that the LRX/RALF/FER signaling concept is a simplified model of the in vivo situation. It is likely that additional proteins are involved in this process and might interact with LRX proteins. Hence, we are investigating possible interaction partners of LRX and study their effects on cell development, cell wall formation, and function of LRX proteins.
The extensin domain of LRXs shows the typical features of hydroxyproline-rich glycoproteins (HRGPs), structural proteins frequently found in plant cell walls. The extensin domain can covalently crosslink in the cell wall, thus insolubilizing the LRX protein, which is important for the function of LRXs.
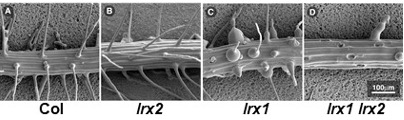
LRX1 and LRX2 of Arabidopsis are predominantly expressed in root hairs and mutations in these genes induce changes in cell wall development that result in aberrant root hair formation. Using the lrx1 mutant root hair phenotype, we are investigating the functional redundancy among the LRX gene family of Arabidopsis. Furthermore, complementation of the lrx1 root hair phenotype was used for a detailed analysis of the extensin domain of LRX1. This work revealed redundancy among the repetitive domains of the LRX1 extensin domain. Extensins are known to form intra- and intermolecular crosslinks via Tyr residues. In the case of LRX1, crosslinking does not solely depend on Tyr residues, suggesting that alternative mechanisms to crosslink LRX1 in the cell wall are present, which remain to be identified.
Selected publications of our group
Baumberger, N., et al., Genes Dev., 2001. 15: p. 1128-1139. https://genesdev.cshlp.org/content/15/9/1128.full
Baumberger, N., et al., Plant J., 2003b. 35: https://doi.org/10.1046/j.1365-313x.2003.01784.x
Draeger, C., et al., BMC Plant Biol., 2015. 15: https://doi.org/10.1186/s12870-015-0548-8
Fabrice, T., et al., Plant Physiol., 2018. 176: p. 1981-1992. https://doi.org/10.1104/pp.17.01374
Herger, A., et al., Curr. Biol., 2019. 29: p. R851-R858. https://doi.org/10.1016/j.cub.2019.07.039
Herger, A., et al., PLoS Genet., 2020. 16: p. e1008847-e1008847. https://doi.org/10.1371/journal.pgen.1008847
Ringli, C., Plant J., 2010. 63: p. 662-669. https://doi.org/10.1111/j.1365-313x.2010.04270.x
Our work was and/or is supported by several grants of Swiss National Science Foundation and the SystemsX/MechanX programs, the University of Zurich, and a Syngenta-Fellowship.
Suppressors of the lrx1 root hair mutant phenotype
EMS mutagenesis of the lrx1 mutant resulted in the identification of a number of rol (repressor of lrx1) mutants that show restored root hair formation. Several of the rol loci were identified and characterized. For two rol mutants, rol5 and rol17, an impact on the TOR (Target of Rapamycin) pathway was found. TOR is a major controller of eukaryotes coordinating growth processes based on growth stimulators and nutrient availability by affecting e.g. translation, mitochondrial activity, and the actin cytoskeleton. Hence, alterations in the TOR network induced in the rol mutants influence the LRX/RALF/FER signaling module, a finding that is confirmed by the direct interaction of the TOR kinase with FER.
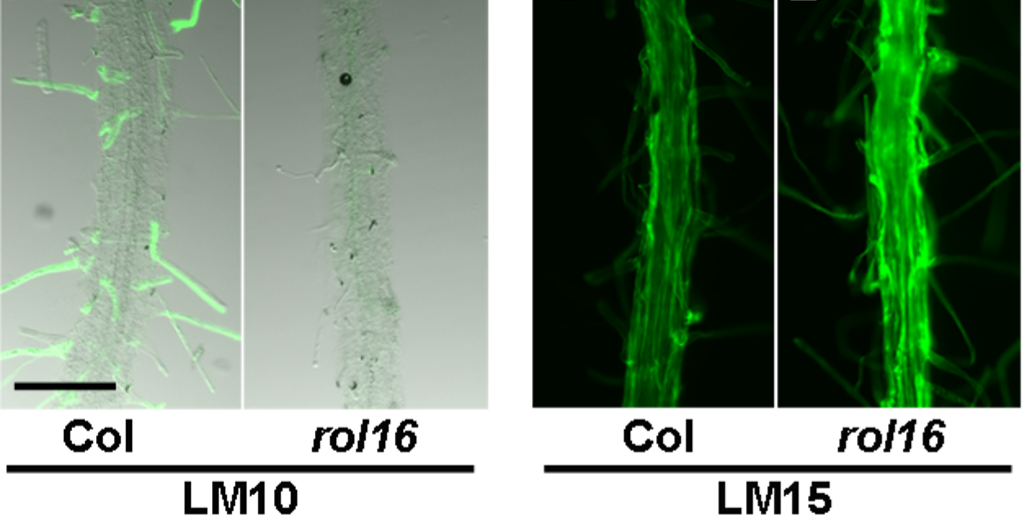
ROL16, at the other hand, codes for a golgi-localized Apyrase that also influences growth properties and cell wall structures. Mutations in ROL16 have a direct effect on cell wall architecture, proposing that ROL16 might be under the influence of the LRX/RALF/FER signaling module.
With our past and ongoing work, we want to identify proteins that are directly or indirectly involved in LRX function to better understanding these proteins and the subsequent signaling mechanisms that are involved in the LRX/FER/RALF signaling module important for cell wall integrity sensing.
Selected publications of our group
Diet, A., et al., Plant Cell, 2006. 18: p. 1630-1641. https://doi.org/10.1105/tpc.105.038653
Gupta, S., et al., PLoS Genet, 2024. 20: p. e1011087. https://doi.org/10.1371/journal.pgen.1011087
Leiber, R.M., et al., Plant Cell, 2010. 22: p. 1898-1908. https://doi.org/10.1105/tpc.109.073007
Schaufelberger, M., et al., J. Exp. Bot., 2019. 70: p. 2313-2323. https://doi.org/10.1093/jxb/ery463
Our work was and/or is supported by several grants of Swiss National Science Foundation, the University of Zurich, and a Syngenta-Fellowship.
The dominant negative effect of the extensin-less LRX1
The extensin domain of LRX1 has revealed to be essential for protein function. Expressing an extensin-less version of LRX1, LRX1ΔE, induces a dominant negative effect on root hair development, resulting in essentially root hair-less seedlings.
This LRX1ΔE-induced root hair defect is used as a genetic tool to identify new components that are able to modify LRX1-related processes. An EMS mutagenesis was performed on an LRX1ΔE-expressing line and a number of suppressor of the dominant-negative effect (sune) mutant were isolated. Several sune loci were identified and the characterization of these loci is currently under way.
Selected publications of our group
Ringli, C., Plant J., 2010. 63: p. 662-669. https://doi.org/10.1111/j.1365-313x.2010.04270.x
Our work was and/or is supported by several grants of Swiss National Science Foundation and the University of Zurich.
Flavonols influence cell growth
Flavonols are plant secondary metabolites that have health-promoting properties and, in plants, influence developmental processes by modulating auxin transport and -accumulation.
rol1 (repressor of lrx1) mutants are affected in the RHAMNOSE SYNTHASE1 (RHM1) and show modifications in rhamnose-containing pectins, important components of the cell wall, and in the glycosylation profile of flavonols. Our work shows that shoot development, particularly cell shape formation in pavement cells, is influenced by the modified flavonol glycosylation. By contrast, root formation including suppression of lrx1, is not under control of flavonols but rather induced by the observed alterations in pectin structures. The mechanisms by which the altered flavonol glycosylation profile influences cell shape formation has been investigated and revealed to be connected but not limited to changes in auxin distribution.
Selected publications of our group
Kuhn, B.M., et al., J. Biol. Chem., 2016: https://doi.org/10.1074/jbc.m115.701565
Kuhn, B.M., et al., Plant Physiol., 2011. 156: p. 585-595. https://doi.org/10.1104/pp.111.175976
Kuhn, B.M., et al., Scientific Reports, 2017. 7. https://doi.org/10.1038/srep41906
Ringli, C., et al., Plant Cell, 2008. 20: p. 1470-1481. https://doi.org/10.1105/tpc.107.053249
Our work was and/or is supported by several grants of Swiss National Science Foundation and the University of Zurich.